- DehydraTECH-oral nicotine delivery peaked in bloodstream 10x to 20x faster than controls
- Peak levels achieved were up to 10x higher than controls
KELOWNA, BC / October 5, 2021 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the “Company” or “Lexaria”), a global innovator in drug delivery platforms is pleased to announce that its recent oral nicotine absorption study NIC-A21-1 revealed that DehydraTECHTM-nicotine delivered via the oral pouch product format required only 2 to 4 minutes to deliver nicotine levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls.
DehydraTECH-nicotine also reached statistically significant peak blood plasma levels up to 10-fold higher overall than controls (p=0.004) while still clearing from blood virtually as quickly as the controls. Lexaria believes that these findings support the world’s fastest-acting nicotine oral mucosal absorption, offering a safer nicotine alternative for the world’s 1.1 billion smokers to kick the antiquated habit. The study utilized Lexaria’s recently developed, advanced “DehydraTECH 2.0” nicotine formulations and was conducted by a leading, independent testing laboratory in the United States.
Lexaria’s DehydraTECH technology does not function with cigarettes or with vape devices, two outdated nicotine delivery methods that cause unnecessary harm. DehydraTECH is instead focused on assisting the world’s smokers and vapers to select less harmful nicotine dosing methods via the oral mucosal absorption route, thereby reducing the carnage caused by smoking.
In the study, the generic nicotine benzoate pouch required approximately 45 minutes to reach its peak delivery rate whereas the DehydraTECH nicotine benzoate pouch reached peak delivery rates at both 8 minutes and again at 30 minutes. In fact, just 4 minutes after the pouch was placed in the mouth, the DehydraTECH-nicotine had reached a higher delivery level than the generic achieved at any point during the study.
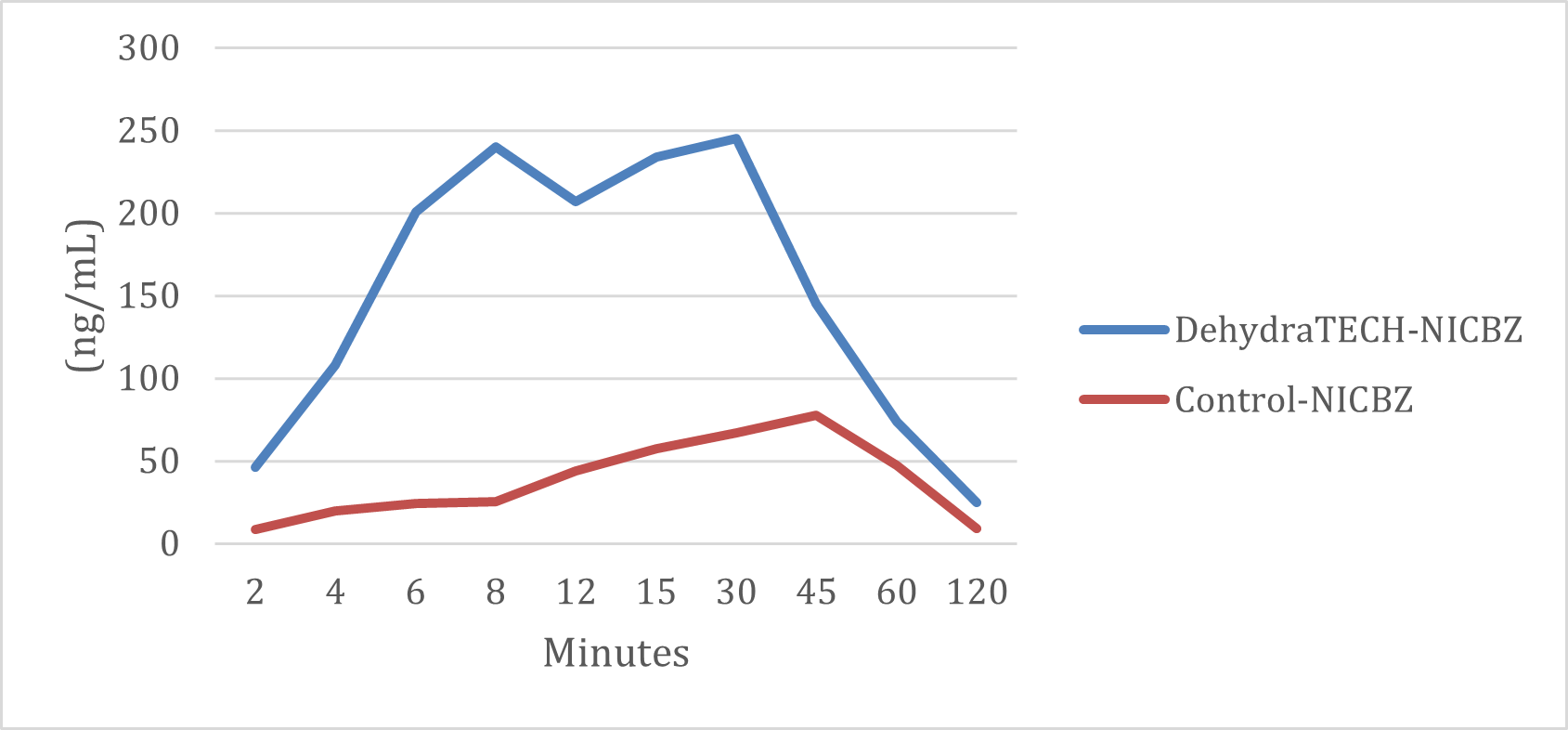
DehydraTECH vs. Control NICBZ Formulations Nicotine Plasma Levels (ng/mL)
Similarly, the generic nicotine polacrilex pouch also required approximately 45 minutes to reach its very subdued peak delivery rate while the DehydraTECH nicotine polacrilex pouch achieved a comparable level in just 2 minutes. The DehydraTECH nicotine polacrilex pouch delivered over 10 times the nicotine level in blood plasma at peak than the generic version.
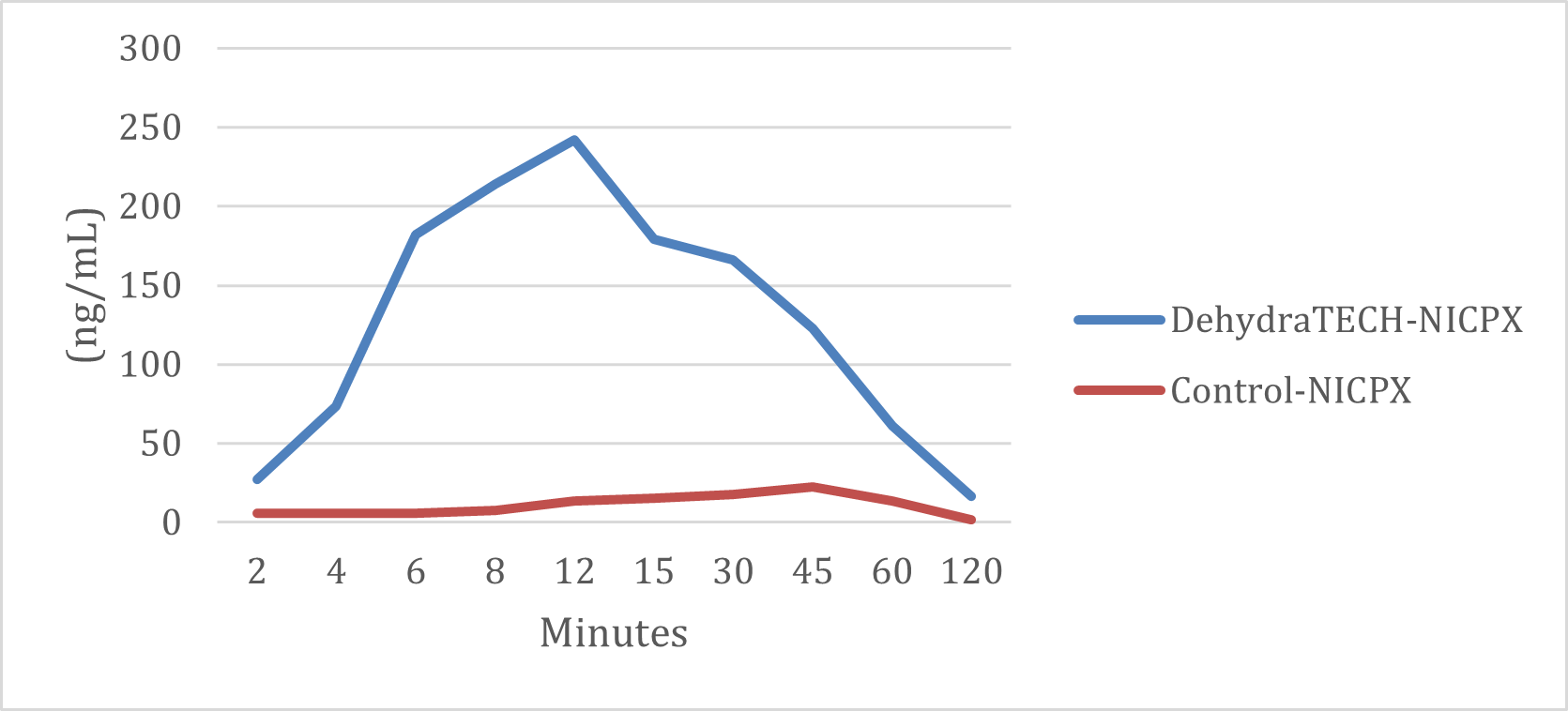
DehydraTECH vs. Control NICPX Formulations Nicotine Plasma Levels (ng/mL)
Key pharmacokinetic findings from the study are tabulated below demonstrating statistically significant improvements in peak and total nicotine delivery (i.e., maximum concentration or Cmax* and total area under the curve up to the point of the last measurement or AUClast** respectively):
|
Nicotine Type |
DehydraTECH Cmax* % Improvement (ng/mL) |
Control (ng/mL) |
DehydraTECH AUClast** % Improvement (hr ng/mL) |
Control (hr ng/mL) |
|
Nicotine Benzoate |
367.3 ± 220.2 263% (p=0.002) |
101.1 ± 39.0 |
227.6 ± 86.2 169% (p=0.0003) |
84.7 ± 13.5 |
|
Nicotine Polacrilex |
344.6 ± 286.7 1052% (p=0.004) |
29.9 ± 15.8 |
179.3 ± 73.6 664% (p=0.00004) |
23.5 ± 8.7 |
“We are extremely pleased with the performance of our latest DehydraTECH-2.0 nicotine oral pouch formulations in this study,” said Chris Bunka, CEO of Lexaria. “Our technology was ten to twenty times faster in delivering comparable levels of nicotine into bloodstream than the peak of the concentration-matched controls and went on to far exceed their total delivery, which should provide much greater consumer satisfaction. Performance gains of this magnitude could be of great significance in enabling the oral pouch product category to offer improved nicotine satiety and effectiveness, with a goal of one day rendering pulmonary administration practices like smoking and vaping as obsolete.”
As a result of the outstanding performance in this nicotine oral mucosal absorption study in animals, Lexaria plans to progress to a larger investigation in human volunteers to compare its DehydraTECH-nicotine pouch performance to that of existing leading brands such as products including Zyn (Swedish Match) and ON! (Altria). Lexaria is currently in the design phase of this proposed human clinical study, which will be independently funded with existing capital. The Company will announce further details as they become available. Lexaria is optimistic that this larger human study will produce positive findings pursuant to those evidenced in its previous 2021 subjective human testing that utilized DehydraTECH-nicotine formulations demonstrating onset of initial nicotine effectiveness in as little as 1.5 to 4 minutes after an oral dose. These results are aligned with the current dog study and compared to an average of 8 to 10 minutes for onset of initial nicotine effectiveness with selected industry products Lexaria previously evaluated.
The global nicotine oral pouch market is among the fastest growing nicotine sectors in the world, with revenues estimated at $2.3 billion in 2020, forecasted to grow to $21.8 billion by 2027. Lexaria believes DehydraTECH is ideally suited for the oral nicotine pouch sector, and that this product format, if empowered with DehydraTECH for rapid and more complete nicotine delivery, could revolutionize the nicotine industry as the first practical, reduced risk category to successfully challenge smoking tobacco. In October 2019, the Food and Drug Administration for the first time ever, authorized reduced risk claims for certain nicotine oral pouches stating that these products “put you at lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis,” compared to cigarettes.
The global tobacco market represents the total revenue generated by all smokers, vapers, and users of all forms of tobacco and nicotine in commercial use today, and is an $818 billion market. Historically, alternative nicotine products have failed to deliver consumer satisfaction to a sufficient degree to replace smoking, in part because of the slowness with which the nicotine enters the bloodstream. In a nicotine industry traditionally slow to innovate, Lexaria believes that its fast-acting DehydraTECH oral nicotine could enable development of the best non-combusted oral nicotine product format to deliver that satisfaction.
Cigarette smoking is one of the world’s biggest preventable health hazards, killing 8 million people per year. Lexaria is focused on oral nicotine products that do not contain tobacco in its leaf form. By comparison, Lexaria regards so-called “heat-not-burn” products and vape devices to be inferior intermediate nicotine formats from the perspective of achieving less harmful outcomes.
About DehydraTECH-Nicotine
DehydraTECH-nicotine is a unique nicotine formulation Lexaria has developed and is optimizing, using tobacco-derived nicotine benzoate or nicotine polacrilex raw materials, based on its patented and proprietary DehydraTECH drug delivery technology. Fast-acting DehydraTECH is designed to improve the way active molecules enter the bloodstream upon oral administration. DehydraTECH has also previously demonstrated enhanced delivery of nicotine into brain tissue, which is of particular importance for improved effectiveness and satisfaction.
The DehydraTECH-nicotine formulations used in the study described herein were based upon Lexaria’s latest “DehydraTECH-2.0” advancements and were tested with average potencies of 3.1 mg to 3.3 mg mg of nicotine per pouch for the DehydraTECH nicotine benzoate pouches, and between 3.5 mg to 3.8 mg of nicotine per pouch for the DehydraTECH nicotine polacrilex pouches. The concentration-matched non-DehydraTECH control pouches used in the study were tested with average potencies of 3.1 mg and 3.5 mg of nicotine per pouch respectively.
About Study NIC-A21-1
Study NIC-A21-1 was performed by a leading, independent testing laboratory in 40 anesthetized male beagle dogs weighing roughly 8-14 Kg each. The dogs were divided into four groups of ten, and each had one of the four study test article nicotine pouches placed in their buccal space while laying on their side for a 30-minute duration. The pouches were uniformly squeezed gently every five minutes over this period and care was taken to ensure that saliva did not escape their mouths through the entire two-hour duration of the study observation period. Blood samples were taken at intervals of 0, 2, 4, 6, 8, 10, 15, 30, 45, 60 and 120 minutes. All dogs were well treated, tolerated the pouches well and returned to full health following conclusion of the study.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.’s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids and nicotine by up to 5-10x, reduce time of onset from 1 – 2 hours to minutes, and mask unwanted tastes; and is also being evaluated for orally administered anti-viral drugs, non-steroidal anti-inflammatory drugs (NSAIDs), and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 21 patents granted and over 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as “anticipate,” “if,” “believe,” “plan,” “estimate,” “expect,” “intend,” “may,” “could,” “should,” “will,” and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company’s ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company’s best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. All recreational nicotine products pose risks to users and should be avoided if possible. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company’s ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company’s public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. There is no assurance that any of Lexaria’s postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/666759/Lexaria-Oral-Nicotine-Study-NIC-A21-1-Delivers-Outstanding-Results