CBD is a substance with poor systemic blood exposure. One scientist explains the challenge and what it means.
Also, could specific lipid-based delivery systems help?
Interest in the therapeutic use of nonintoxicating cannabinoids and other hemp-derived substances—particularly cannabidiol (CBD), cannabigerol (CBG), and their carboxylic analogs—has reached fever pitch in recent years as CBD-containing products appear everywhere, from online retailers to grocery stores and gas stations1.
The widespread availability of CBD products is a cumulative effect derived from substantial progression in the scientific research over the past 30 years as the many active ingredients of hemp strains were isolated and major discoveries were made regarding the endocannabinoid system (ECS)2. In the United States, CBD received further validation when the U.S. Food and Drug Administration (FDA) approved the proprietary drug Epidiolex as adjunctive therapy (not a monotherapy) for pediatric patients with drug-resistant epilepsy. CBD is an active pharmaceutical ingredient in Epidiolex.
Although the international medical community became more open to recognizing the tentative functionality of the ECS in maintaining the body’s homeostasis and immune responses3, the published papers on the direct quantitative correlation between CBD cellular pharmacology and therapeutic efficacies indicate that the popularization of many CBD-fortified or CBD-labeled health products, and associated health claims, lack a rigorous scientific foundation and integrity1,2. As a matter of fact, the formal monograph for Epidiolex clearly indicates that the precise mechanisms by which Epidiolex exerts its anticonvulsant effect in humans are unknown. Clinicians and pharmacologists concluded that Epidiolex does not appear to exert its anticonvulsant effects through interaction with cannabinoid receptors4.
Consequently, the reputation of CBD or other phytocannabinoids as a universal “cure-all” puts those important and promising substances in the same class as other “natural” panaceas where anecdotal cases and overwhelmingly biased testimonials supersede the proper clinical evaluation of already marketed CBD formulations and finished products.
The broad-ranging therapeutic utility of CBD has been attributed to its reported pharmacological activity at a number of target receptors, such as cannabinoid receptor type 1 and 2 (CB1 and CB2), transient receptor potential (TRP) channels, and G-protein-coupled receptor (GPR55), to name a few2,5. From a careful inspection of the original publications where these bioactivities are reported, it’s possible they are irrelevant to many non-prescription products given the relatively small dose of CBD that many such products contain and the low systemic exposure of CBD in human subjects.
Bioavailability Challenge
Oral bioavailability and exposure, together with systemic clearance (metabolic and unchanged substance excretion), Cmax (maximum blood concentration), volume of distribution, and terminal half-life (t1/2) are the principal pharmacokinetic properties of bioactive compounds. Poor oral bioavailability and an inferior systemic exposure are two of the leading causes of failures in clinical trials and/or therapeutic evaluations of initially promising potent compounds. This leads to hindering the estimation of what dose will be needed to support the treatment of certain medical conditions2,5. Also, an extremely low bioavailability of CBD oil leads to highly variable exposures between individuals—studies show around a 20-fold difference—which is not acceptable if we want to be able to use CBD in a correct therapeutic manner5,6.
Absolute oral bioavailability refers to the fraction of the drug available to the body or system. This is measured as a ratio between the blood concentration-time-curves (usually as area under the curve, or AUC) after oral (per os) and intravenous (IV) administration. Absolute bioavailability cannot exceed 100% since it is assumed that 100% of the drug is available to the body after IV administration. AUC and exposure are two terms used interchangeably.
According to the principles of pharmacokinetics, as soon as the substance molecule enters the blood circulation after either intravenous or oral administration, it is subjected to tissue distribution and hepatic/bile/renal clearance. For the substances that are extensively metabolized and cleared (as studies show CBD is), the blood AUC values after IV or PO administration are low as well. Therefore, CBD oral bioavailability (%F), estimated by the AUC(PO) / AUC(IV) ratio, may look acceptable for certain formulations (such as lipid nanoparticle, polymers-based, etc.), but in general it leads to the erroneous and misleading interpretation concerning the therapeutically meaningful oral exposure of CBD that can actually provide high enough blood concentrations of CBD to engage and inhibit/activate specific receptors and enzymes that are key to reaching therapeutic, wellness-associated efficacies6.
For instance, following a daily administration of 10 mg/kg (~800 mg/day) of CBD in 15 neuroleptic-free patients for 6 weeks, the mean CBD plasma level ranged from 11-36 ng/ml (less than 100 nM). However, CBD only displaced the agonist from the majority target receptors at the concentration of 16-32 µM. In other words, the in vivo plasma levels need to be more than 100-200-fold higher to inhibit and modulate those receptors6,7.
Why It Matters
Based on the available commercial literature, CBD is being touted as a remedy for addiction, aging, anxiety, arthritis, concussion, depression, nausea, obesity, pain, Parkinson’s disease, post-traumatic stress disorder, and psychiatric disorders, among others. However, no evidence of double-blinded, placebo-controlled clinical trials that supported these claims could be located by the author, and anecdotal claims of CBD therapeutic utility often arise from what are actually inconclusive studies6-8.
Nevertheless, many CBD companies purportedly ignore this fact in an attempt to rationalize the commercial utility of phytocannabinoids and exploit the “natural” label, without any R&D efforts to characterize their marketed products structurally, pharmacokinetically, and, ultimately, therapeutically, which would otherwise establish a certain level of evidence-based credibility for the aforementioned marketed products1,2.
Although CBD and other hemp-derived phytocannabinoids remain a formally unproven therapeutic option, with little known about their mechanism of action, physicians and holistic health/wellness practitioners still remain open to the possible future role these products could play in the field of alternative management and prophylaxis of different health conditions, such as chronic inflammation and pain1,2.
In order to recommend a particular phytocannabinoid-based product to patients and wellness-oriented end-users, those practitioners need to adequately analyze specific information beyond of the certificate of analysis, such as an experimentally assessed systemic bioavailability and in vivo targeted efficacy of a given product. A very brief survey of the published scientific and commercial papers or conference presentations indicates that many companies in the CBD market do not possess such information to differentiate and promote their products based on at least preclinical and/or exploratory clinical study reports2,3,6.
On the other hand, this particular status quo that is postulated by the deficiencies in pharmacokinetic/pharmacodynamic correlations, and substantiation of cannabinoid-containing nutraceutical products, represents the limitless opportunities for R&D-driven nutraceutical and general wellness-oriented companies in an everchanging cannabinoid market landscape. Physicians and holistic health/wellness practitioners are looking for more advanced, research evidence–based cannabinoid products with high, unparalleled, and, first and foremost, unbiased expectations of achievable health benefits.
Can Lipid Deliveries Help?
Hemp-derived CBD is a highly lipophilic, small-molecule-size natural substance that is intrinsically insoluble in water. (See Figure 1.)
Figure 1: Physicochemical properties of CBD. Courtesy of Levan Darjania, PhD
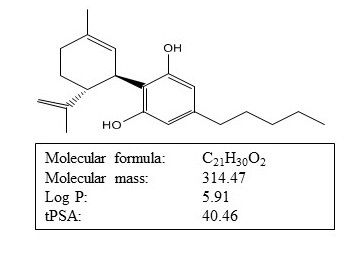
For lipophilic compounds, intestinal lymphatic transport may be highly efficient and the predominant route of transport to the systemic blood circulation following oral delivery. For these substances, lymphatic access occurs via association with lipid absorption and lipoprotein assembly pathways during diffusion across intestinal absorptive cells (enterocytes). Upon exocytosis from enterocytes, drug-lipoprotein complexes are transported across the basement membrane and are trafficked from the intestinal lamina propria via the lymphatics9. It was demonstrated that the physicochemical properties required to promote drug association with intestinal lipoproteins—and therefore to promote lymphatic transport—were a logP value of >5 and substantial solubility in long-chain triglyceride (LCT)7,9.
A research group from the University of Nottingham in the UK reported that oral administration of CBD with LCT lipids can substantially increase the intestinal lymphatic transport in disease-specific animals. The concentration of CBD recovered in mesenteric lymph nodes was profoundly higher than that observed after administration with non-LCT formulation and as much as 250-fold higher than the concentration in plasma6. This is mainly attributed to the fact that compound delivered to the intestinal lymph avoids first-pass metabolism as lymph drains directly into the systemic circulation via the thoracic lymph. That results in the lymphatic concentrations of hydrophobic compounds orders of magnitude being higher than that attained in systemic blood6.
In addition, CBD showed potent immunomodulatory effects at ~15 μM by suppressing the intracellular profile and expression of proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, and IL-17A in animal and human T cells (residing in the mesenteric lymph nodes)6,7. Although the suppressive potency of CBD to attenuate pathogenic cytokine released by T cells was only observed at micromolar concentrations, this concentration is achievable in the intestinal lymphatic system, but not in blood circulation6,7.
The intestinal lymphatic system is the major host of immune cells. Therefore, super-high levels (>300 μM) of CBD in the lymph can lead to prominent immunomodulatory effects such as inhibiting lymphocytes proliferation and the expression of inflammatory cytokines from T cells. These two are key pathogenic cells present in a wide range of autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, allergic asthma, and inflammatory bowel disease2, 5-7.
R&D Focus and Product Development Strategy
Biomedical discoveries about the ECS thus far clearly indicate that it is an important target for the development of nutraceutical products addressing a variety of health conditions. However, there are substantial challenges in determining the right balance of cannabinoid components in order to establish a defined product with which to move forward in consistent and efficacious product development. Referred to as the “entourage effect,” unique therapeutic effects of heterogeneous extracts from hemp are hypothesized to be achieved through a complex synergy between cannabinoids and the many other secondary constituents of the plant1,5,6. The limitations of the entourage effect are that, at this time, it is not clear which compounds drive the effect, which pharmacodynamic effects of cannabinoids are impacted, or whether this can be standardized for improved and consistent cannabinoid therapeutics1-3.
Investigating individual cannabinoids, terpenoids, and their combinations has merit over investigating heterogenous full- and broad-spectrum hemp extracts mainly because this pathway is consistent with how most other pharmaceutical medications are currently developed. Therefore, for science-based companies, there are clear criteria for the discovery, evaluation, development, and quality control of consistently efficacious nutraceutical products and remedies according to the established pharma-industry standards.
Discovery and development of highly consistent and efficacious cannabinoid-based products is a global task that is based on multidisciplinary approaches, collaborations, and partnerships. Therefore, technology-driven companies need to get involved in building their collaborative platform with academic institutions and contract research organizations to utilize well established ECS-target biochemical screening models and develop customized wellness products addressing ECS deficiencies. The companies and their R&D team need to develop innovative nutraceutical products and brands by reconstituting an entourage effect through the synergistic efficacy studies of individual cannabinoids. This could be outlined as follows:
- Which major/minor cannabinoids, terpenoids, flavonoids, etc., are the most potent hits to engage with the immune disease–specific targets to achieve lowest half maximal inhibitory concentration (IC50)?
- When initial “biochemical” IC50 are established, there is a need to check whether those selected phytocannabinoids and terpenoids are not hitting badly some housekeeping genes and enzymes in order to avoid some obvious off-target toxic and unwanted side effects.
- Later, optimal “biochemical” composition should be tested in vitro or ex vivo utilizing specific cell or tissue cultures to ensure that individual “potent” components can permeate cell membranes and reach intracellular ECS receptors at concentrations determined in the preliminary biochemical IC50 assays.
- In the case of positive results, the properly adjusted composition (formulation) needs to be tested pharmacokinetically (PK) in disease-model animals to ensure that the very same formulation components can reach lymphatic and/or systemic circulation after oral (or pulmonary) administration.
- Very often, very potent substances remain as “potent substances” and never become therapeutic remedies because of poor PK profiles. Thus, certain formulation adjustments (qualitative and quantitative) are needed to achieve the optimal systemic exposures of substances of interest.
- When a favorable PK profile is established, one needs to test the very same formulation in disease-specific animals to ensure that the desired therapeutic effect is achievable in humans.
- And finally: when all the above is successfully tested, properly designed clinical evaluation and substantiation in human subjects should be conducted.
In summary, while there appears to be tremendous therapeutic potential for cannabinoid health- and wellness-associated products, there is a need for the development of defined, consistent, and targeted products with established standards for quality, safety, and efficacy before being recommended for use. The ultimate goal is to develop and promote scientific evidence–based ECS-modulating cannabinoid products that will be trusted by medical communities, physicians, holistic health practitioners, and wellness-oriented end users.
Levan Darjania, PhD, is chief scientific officer at Green Hygienics Holdings Inc. (Poway, CA), with more than 26 years of R&D experience in biotechnology and pharmaceutical drug development.
References
- Bonn-Miller MO et al. “Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches.” International Review of Psychiatry, vol. 30, no. 3 (June 2018): 277-284
- Nelson KM et al. “The essential medicinal chemistry of cannabidiol (CBD).” Journal of Medicinal Chemistry, vol. 63, no. 21 (November 12, 2020): 12137-12155
- Hanus LO et al. “Phytocannabinoids: a unified critical inventory.” Natural Product Reports, vol. 33, no. 12 (November 23, 2016): 1357-1392
- FDA access data. Epidiolex. Reference ID: 4282447
- Bih CI et al. “Molecular targets of cannabidiol in neurological disorders.” Neurotherapeutics, vol. 12, no. 4 (October 2015): 699–730
- ZgairA et al. “Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation.” Nature Scientific Reports, vol. 7, no. 1 (November 6, 2017):14542
- Gershkovich P et al. “Different impacts of intestinal lymphatic transport on the oral bioavailability of structurally similar synthetic lipophilic cannabinoids: dexanabinol and PRS-211,220.” European Journal of Pharmaceutical Sciences, vol. 31, no. 5 (August 2007): 298-305
- Leehey MAet al. “Safety and tolerability of cannabidiol in Parkinson disease: an open label, dose-escalation study.” Cannabis and Cannabinoid Research, vol. 5, no. 4 (December 15, 2020): 326–336
- Trevaskis NLet al. “From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity.” Nature Reviews. Drug Discovery, vol. 14, no. 11 (November 2015): 781-803
Kratom sees impressive sales growth despite its regulatory status and stigma
March 12th 2025Despite its controversy, kratom is a top-selling ingredient that consumers see value in. That said, brands need to work hard to demonstrate safety and quality of kratom products in the absence of legal regulatory status. Will kratom be able to overcome its stigma for even more growth and consumer acceptance?







