Technology


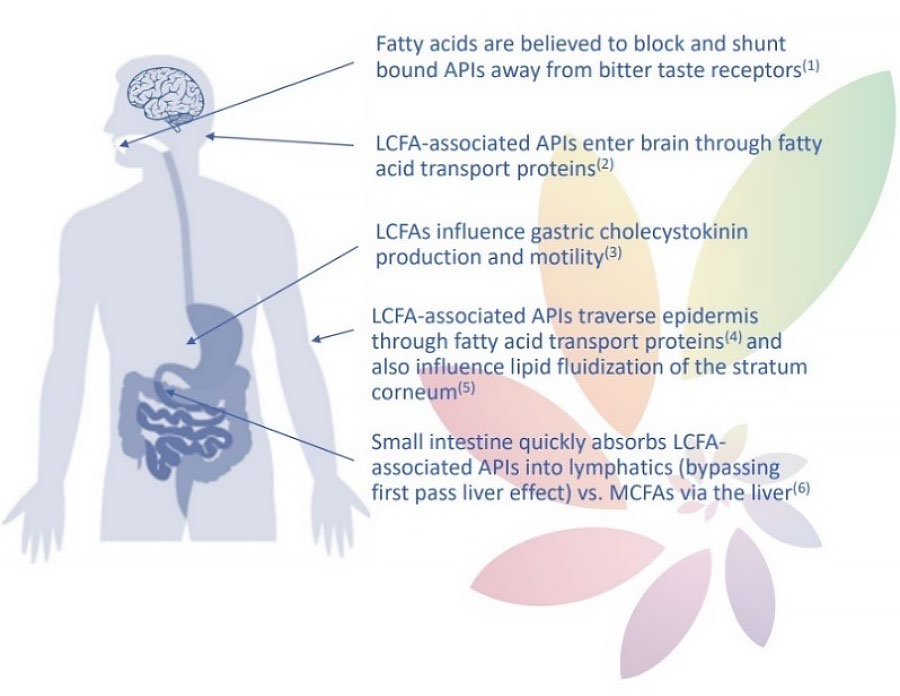
Commercial Applications
Lexaria’s DehydraTECH is designed specifically for formulating and delivering lipophilic (i.e., fat-soluble) drugs and active ingredients. DehydraTECH is protected by a robust suite of pending patents or patents already granted around the world that cover its use with a broad range of bioactive molecules.
Lexaria’s technology is best thought of as an additional layer that improves the effectiveness of existing or planned new products for companies that offer prescription and non-prescription-based drugs. Lexaria licenses its advanced technology to other companies around the world to offer consumers the best performance possible across an array of product formats.
Lexaria’s ongoing R&D is mainly focused on development of product candidates across various key segments.

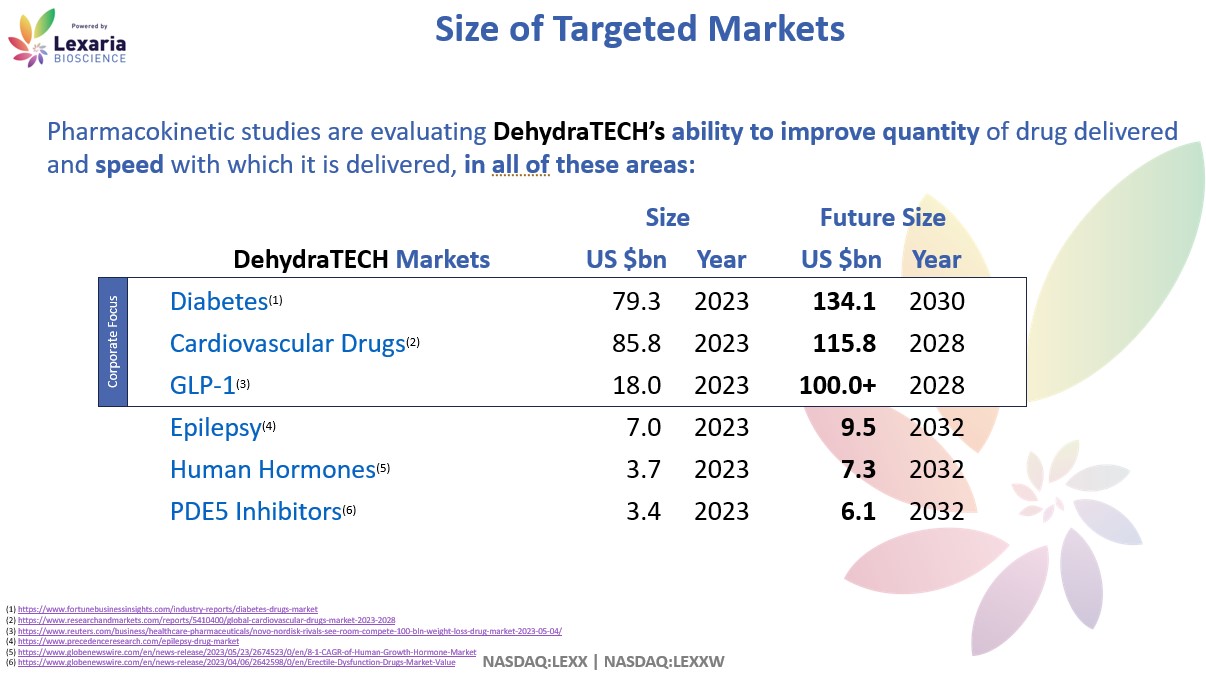
Pharmaceuticals: Hypertension and Heart Disease
The current global market size for drugs used to treat various heart diseases is $96.1 billion, and anticoagulants, antihypertensive, and antihyperlipidemic drugs comprise roughly 90% of these. The impact of COVID-19 on corporate operations led to recent growth in the market, which is expected to reach $107.77 billion by 2025.
Cardiovascular disease caused an estimated 17.9 million deaths around the world during 2019, and in the U.S., nearly one-half of all Americans suffer from some form of cardiovascular disease. Lexaria has already demonstrated in a pilot human clinical study, the ability to statistically significantly decrease human blood pressure, theorized to have occurred as a result of the known vasodilatory potential of our substance of choice. The generic version is known to have poor absorption characteristics, such that typically only about 6% of what is orally ingested actually finds its way into blood circulation. Research has shown that Lexaria’s processing technology increases absorption by 100% to 500%. Lexaria’s DehydraTECH is currently patented in the European Union and Australia for treatment of heart disease.
Pharmaceuticals: GLP-1 Drugs
Rybelsus (semaglutide) is the only GLP-1 drug approved by the FDA for oral dosing to treat diabetes and weight loss. The FDA has also approved semaglutide marketed as Ozempic® and Wegovy®, administered by injection, to treat diabetes and weight loss. All three of these drugs are owned and manufactured by Novo Nordisk®.
GLP-1 drugs have recently been approved by the FDA for type two diabetes and weight loss management. Weight loss of between 10 pounds to 33 pounds, or more, has been widely reported. One 68-week study of 667 people reported an average loss of 15% of body weight.
Anecdotal commentary also suggests that some patients are experiencing reduced cravings for alcohol, nicotine and opioids while taking GLP-1 drugs. Other trials are examining their effects on heart disease and even dementia in part because of evidence that GLP-1 drugs may reduce the build-up of the proteins amyloid and tau in the brain, thought to be partly responsible for Alzheimer’s disease.
Side effects of GLP-1 drugs vary but can include nausea, vomiting, diarrhea and more. A small number of GLP-1 drugs have already been tested or approved in oral format but some studies have reported worse side effects with the oral form. The drugs are also being investigated for their relationship to bone density, muscle loss and more. Because of potential serious side effects, it may be beneficial to treat patients with lower oral doses of the drugs, something that Lexaria’s DehydraTECH technology may enable if it can improve the PK performance of GLP-1 drugs through oral capsules.
Because GLP-1 drugs have experienced FDA approvals as recently as 2021 and 2022, and because the health benefits of this drug class are still being discovered and understood, the potential market size is unknown. Published reports are widely estimating $100 billion in sales per year, by 2030. At least one analyst from Guggenheim Partners published a note on September 12, 2023 in which he explained how “the total addressable market for these so-called incretin drugs could balloon to $150 billion to $200 billion.”
Pharmaceuticals: Other Pharmaceutical Areas of Focus
Our Lexaria Pharmaceutical Corp. subsidiary has worldwide rights to our patent portfolio related to pharmaceutical applications, including a broad range of active molecules such as glucagon-like peptide (GLP-1) drugs, antiviral drugs, hormone treatments utilizing estrogen or testosterone, treatments for dementia, rheumatoid disease, and phosphodiesterase inhibitors (PDE5).
Lexaria’s technology significantly enhances oral delivery of certain antiviral drugs. To date we have evidenced that DehydraTECH improves the delivery of each of the following drugs into animal bloodstreams: darunavir, efavirenz, remdesivir, ebastine, and cholchicine; all of which are known to possess various antiviral properties.
Meanwhile, an in vitro screening assay determined that DehydraTECH-enabled remdesevir and ebastine were effective in inhibiting the COVID-19 SARS-CoV-2 virus.
Viral-caused diseases such as HIV, hepatitis, influenza, and coronavirus remain common and at times reach pandemic proportions. Hepatitis causes 1.5 million deaths each year; 35 million cases of influenza were reported in the 2019-2020 flu season in the US alone; and nearly 203 million cases of COVID-19 were recorded through 2021. Additionally, there are currently 37.7 million people worldwide infected with HIV/AIDS.
Vaccines help prevent the transmission of – but do not treat – viral diseases and generally have effectiveness rates that range between 50% and 90%. Antiviral drugs will always be needed to treat people who become infected and are at risk of serious health consequences with viral diseases. In many cases, these drugs are the difference between life and death. The antiviral drug market is expected to experience compound annual growth of 4.6% to reach $77.07 billion by 2028.
Many antiviral drugs are currently administered via injection, a process usually requiring a healthcare professional that introduces expense and complication for mass dosing. Lexaria believes it is possible for DehydraTECH to improve delivery performance such that some of these drugs could be dosed via oral tablet or capsule, which is less expensive and makes it easier and cheaper to treat more patients in less time.
According to market research, the global pharma industry was worth $1.5 trillion in 2022, and over $200 billion was spent on R&D in 2020. This is a larger R&D expenditure than the entire gross revenues of many other business sectors. Lexaria is pursuing or investigating multiple pharmaceutical related applications of DehydraTECH with bioactive molecules that act upon human CB1 and CB2 receptors. Substances that act upon these receptors in the human body that affect pain, inflammation, anxiety and depression have been shown to have effect against cancer and neurodegenerative conditions.